discover devices that enable anyone, anywhere to collect a microsample
Advance RNA research with the GenTegraRNA-NEO Active Chemical Protection™ compound, developed for Mitra® microsampling devices.
Accelerate metabolomics with the Metabolon Global Discovery Panel, validated on Mitra® blood microsamples for efficient bioanalysis.
We offer a Microsampling Resource Library of published articles & resources on microsampling studies. Peruse 400+ peer-reviewed resources.
collect microsamples in the lab, at home, in the field or facility
Eliminate the challenges of traditional specimen collection for science & medicine* with remote devices based on advanced microsampling technologies that deliver convenience with high precision for reliable data & insights.

Easily collect up to 120 µL of blood and transport by mail.

This volumetric sampling device overcomes many DBS issues.

Harpera™
A research tool in development for less invasive skin biopsies.

Our lab tools help with accessing
& processing microsamples.
microsampling has endless applications
You can apply our personalized sampling solutions to advance research and healthcare.
Leverage microsampling for animal studies & the 3Rs of animal research.
Offer remote sampling options to improve trial success rates.
Collect samples easily from diverse cohorts, including adult & pediatric subjects.
Facilitate remote monitoring of study subjects with at-home sample collection.
Apply microsampling in forensics, PEth monitoring of alcohol & anti-doping.
Use remote collection for disease, serology & epidemiology studies.
Combine glass capillaries + DBS for precision that overcomes hematocrit issues in blood sampling.
Enable biological wellness with samples that reveal environmental exposure & other factors.
Analyze omic data from a precise volume microsample of bio-fluid.
oceans of data from just a few drops
microsampling technology with scientific precision and practical simplicity that provides accurate specimen collection for enhanced analysis across different industries.

Tech Resource Library
Articles, Bridging Studies & More

Microsampling Products
Devices, Kits & Lab Automation
Research Support
Supporting Select Innovators









join the microsampling movement embraced by top organizations and scientific innovators around the world

Inventor Spotlight
“This microbiopsy tool is designed to take skin samples much more easily than a traditional biopsy and is virtually painless…”
– Tarl Prow, PhD, Co-Inventor, University of Queensland
Australian researchers Tarl Prow, PhD, H. Peter Soyer, MD, and Alex Ansaldo, MD collaborated at The University of Queensland on the invention of the Harpera™ skin microbiopsy tool to reduce scarring and replace painful skin biopsy procedures in dermatology.
Read the Interview>>

CRS Spotlight
“We have validated more than 12 assays using Mitra technology in support of a wide range of drug applications & study types.”
– Jeff Plomley, MSc, Scientific Director, Altasciences
Altasciences is a clinical research services (CRS) organization providing the accurate and precise drug concentrations sponsors require to support regulatory submissions for both nonclinical and clinical development programs. Altasciences is a recognized leader in microsampling analysis.
View the Lab Directory Profile>>

Product Spotlight
“GenTegraRNA-NEO is an innovative solution that safeguards RNA integrity in dried blood and opens new avenues for RNA-based research by enabling remote, convenient, minimally invasive blood collection methods.”
–Robert (Bob) Barrett, President, GenTegra
GenTegra developed an Active Chemical Protection™ compound that can be applied to VAMS® tips on Mitra® devices prior to blood sampling for RNA-based studies.
View the Product Page>>

… sample collection acceptance rates based on remote specimen collection by end-users

… published materials that demonstrate the scientific accuracy and validity of microsampling.
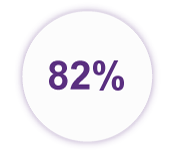
… of people surveyed prefer nearly painless finger-stick sampling vs. stressful needle draws.

… countries where research organizations are publishing on their microsampling studies.
innovate your approach to
science and research
Advancing to microsampling begins with a conversation. Contact us!
1 (310) 787-8747 | info@neoteryx.com


