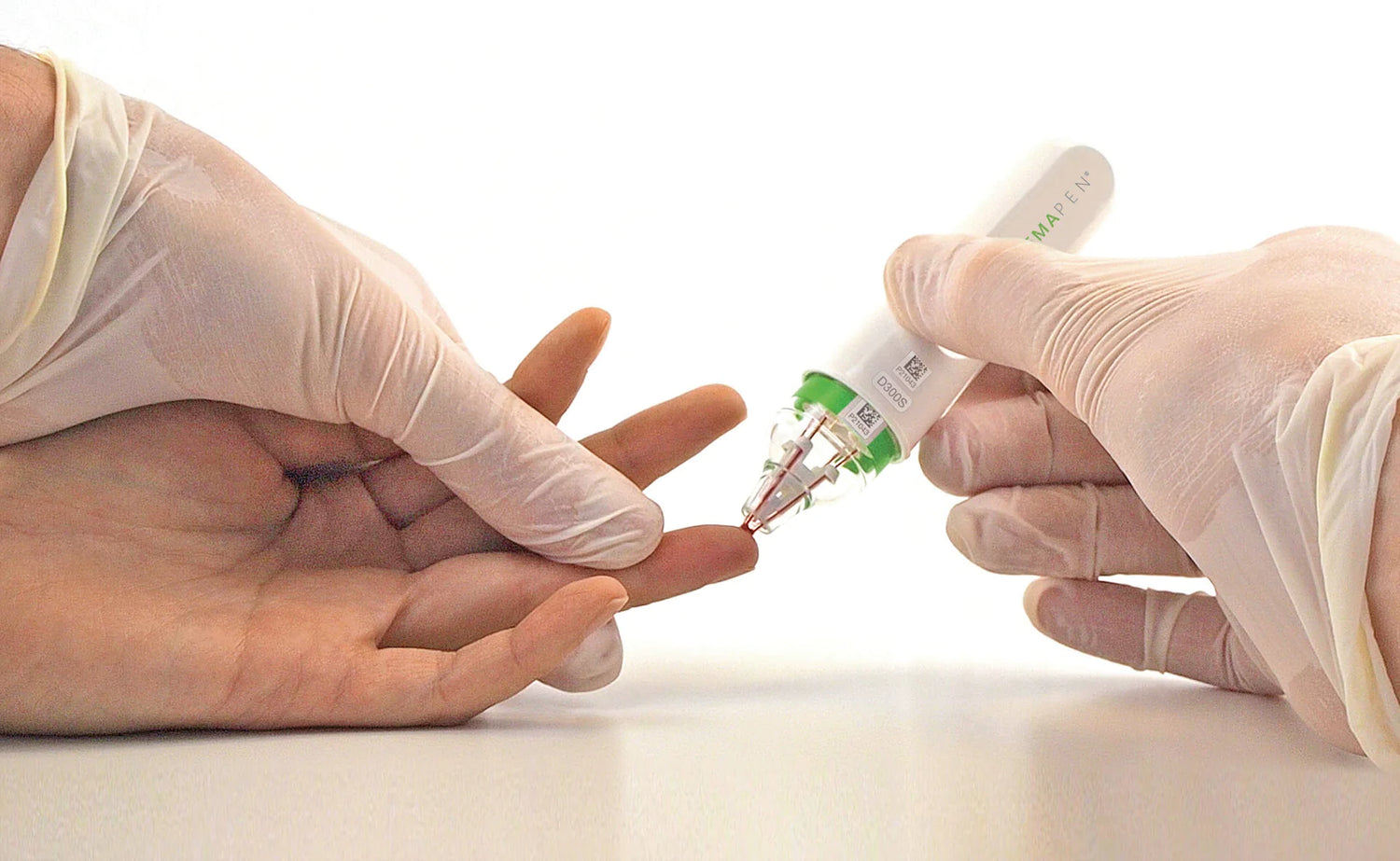
Trajan Scientific and Medical’s hemaPEN® device has been included on the Australian Register of Therapeutic Goods (ARTG). The device enables the volumetrically accurate collection and storage of four dried blood spot (DBS) samples remote to the clinical setting, to drive information-rich healthcare decision making.
Trajan’s Group CEO and Chairman, Mr Stephen Tomisich says, “We believe the future of healthcare is preventative, personal, and will be increasingly based on analytical data. The hemaPEN design is all about delivering robust and reproducible blood sample collection in remote settings. Having confidence in results then allows for informed decision making, especially if we can identify trends over time or across populations. When there is variation in the underlying data it makes identification of the trends far more difficult.”
This first version of the hemaPEN platform is just one of an evolving class of microsampling devices being developed by Trajan.
Microsampling technology is expected over time to deliver real benefits to society, enabling:
- Less invasive and pain-free blood collection compared with phlebotomy.
- More frequent and/or smaller blood volumes to be collected.
- Remote patient testing for many patient demographics.
- Samples to be collected by anyone in any place, and eliminates the need for cold chain transportation.
- Significant time and cost savings for healthcare providers, by reducing collection center costs, transportation fees, storage costs of samples, without compromising patient results.
Mr Tomisich said, “We are witnessing the transition towards more sensitive and selective analytical techniques into clinical pathology.”
hemaPEN has had in-depth evaluations by independent laboratories, and features in a growing number of scientific publications demonstrating its analytical performance, including addressing the limitations of traditional DBS technology.
A recent publication in Analytical Chemistry by toxicology scientists at the University of Ghent in Belgium, concluded that bioanalytical methods can be successfully developed and validated in accordance with the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) using samples collected by the hemaPEN.*
Dr Anne Collins, Trajan’s Business Unit General Manager, Microsampling, commented, “For inclusion on the ARTG, Trajan has declared compliance with the TGA regulatory requirements for safety and performance of hemaPEN, which alongside the independent validation of the analytical performance creates a unique opportunity in Australia and New Zealand, to enable the collection of blood samples that either couldn't or wouldn't normally be taken.”
Since hemaPEN was first introduced for research use worldwide, Trajan has worked closely with its partners in healthcare, pathology, pharmaceutical, food and environmental testing laboratories; with the aim to develop patient-centric technology to improve the blood sampling experience for:
- Those who require frequent testing (e.g. therapeutic drug monitoring to better manage health prognosis).
- Individuals with limited access to healthcare facilities or clinics.
- Patients who are vulnerable to the complications of frequent or large volume blood sampling, such as young children and the elderly.
- Participants in drug development clinical trials, longitudinal studies, or biomonitoring.
“We are working on registering hemaPEN in each major global market.” said Dr Collins.
Trajan works one on one with partners and customers to ensure successful adoption by clinicians and smooth integration into existing laboratory workflows, while introducing all the benefits of hemaPEN technology.
“As a platform technology, there is enormous potential for customization and large-scale deployment of hemaPEN.” said Dr Collins.
Visit www.hemapen.com to purchase or learn more about hemaPEN, and to sign up for updates.
To learn more about Trajan’s microsampling technologies and capabilities visit www.trajanscimed.com/microsampling.
 *Deprez, S. et al. “Evaluation of the Performance and Hematocrit Independence of the hemaPEN as a Volumetric Dried Blood Spot Collection Device.” (Analytical Chemistry 2019 91 (22), 14467-14475)
*Deprez, S. et al. “Evaluation of the Performance and Hematocrit Independence of the hemaPEN as a Volumetric Dried Blood Spot Collection Device.” (Analytical Chemistry 2019 91 (22), 14467-14475)
 Downloads
Downloads
Press release [PDF]
More information
www.hemapen.com
Media contact information
Trajan Scientific and Medical
Tel: +44 (0) 1908 568 844
media@trajanscimed.com
This information is relevant for hemaPEN supplied in Australia and New Zealand only. For other areas please click here.
ARTG number: 280007