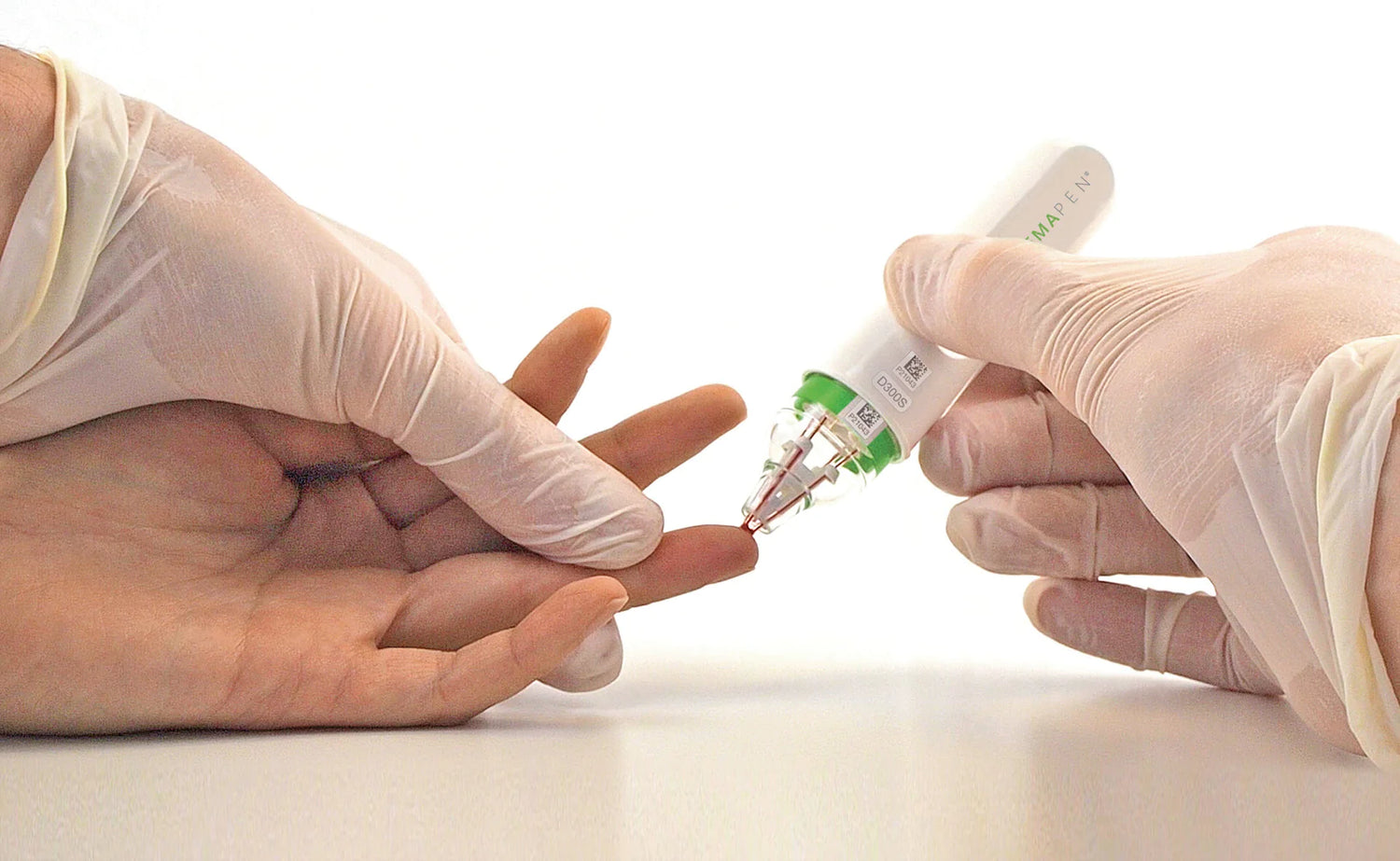
Trajan Scientific and Medical’s (Trajan) hemaPEN® has been independently evaluated and the results of the study published in the Royal Society of Chemistry’s journal Analyst - selected for the cover of issue 17. hemaPEN demonstrated significant advantages for blood sampling to support better healthcare decision making.
What sets hemaPEN apart from other blood microsampling methods, is its easy-to-use pen-like design and the ability to provide volumetrically accurate and precise samples for analysis.
The independent study, proving the hemaPEN methodology to be valid and reliable for application to therapeutic drug monitoring (TDM) of psychiatric patients treated with antidepressant drugs used to manage major depressive disorder, obsessive-compulsive disorder and eating disorders. The study was undertaken by the Research Group of Pharmaco-Toxicological Analysis (PTA Lab), headed by Professor Laura Mercolini at the Department of Pharmacy and Biotechnology (FaBiT) of the Alma Mater Studiorum - University of Bologna, Italy.
The study found that hemaPEN will only collect a volumetrically accurate and precise volume. Even when trying to over or underfill the device by holding it in the sample for longer or shorter periods of time, it was only possible to collect the defined volume.
Researcher Dr Michele Protti said "The original methodology developed in this work proved to be a promising tool for volumetric sampling of capillary whole blood from patients undergoing pharmacological treatment with central nervous system (CNS) drugs.”
“hemaPEN also has high potential for future implementation in self- and home-sampling procedures, paving the way toward patient-centric precision medicine and therapy personalization within the framework of psychiatric pharmacological regimens and neuro-degenerative diseases." said Dr Protti.
The current challenge with at-home microsampling for TDM is the ability to collect quality samples for laboratory analysis. Inaccurate volume sampling can lead to unreliable results and poor healthcare decision making – particularly for monitoring patients over extended periods of time, or for drugs with a narrow therapeutic index.
“hemaPEN was developed to provide a foolproof sample collection procedure, so even if you make mistakes, any samples you did collect will still be volumetrically accurate and precise.” said Dr Andrew Gooley, Chief Scientific Officer, Trajan Scientific and Medical.
“It is great to see a growing number of independent studies taking advantage of hemaPEN blood microsampling; and it is also available for therapeutic and diagnostic use in the EU and UK, and is listed as a Class I IVD by the US FDA and TGA ARTG.” said Dr Gooley.
 The published study method was validated according to European Medicines Agency (EMA) and the U.S. Food and Drug Administration (US FDA) guidelines and is available open-access from Analyst.
The published study method was validated according to European Medicines Agency (EMA) and the U.S. Food and Drug Administration (US FDA) guidelines and is available open-access from Analyst.
Trajan believes in science that benefits people – creating portable and affordable measurement solutions, enabling accurate results to inform preventative healthcare.
Trajan believes in science that benefits people - creating portable and affordable measurement solutions, enabling accurate results to inform preventative healthcare.
Visit www.hemapen.com to purchase or learn more about hemaPEN, and to sign up for updates.
To learn more about Trajan’s microsampling technologies and capabilities visit www.trajanscimed.com/microsampling.
 Download
Download
Press release [PDF]
Related news
The third leg in at-home healthcare – microsampling
US FDA listing of Trajan’s hemaPEN® blood microsampling device
CE mark for Trajan’s hemaPEN blood microsampling device, now available for diagnostic use across EU and UK
Trajan’s hemaPEN included on TGA’s ARTG as first blood microsampling device for use in Australia
European patent for Trajan’s hemaPEN
More information
www.hemapen.com
rsc.li/analyst
Media contact information
Trajan Scientific and Medical
media@trajanscimed.com