7 products
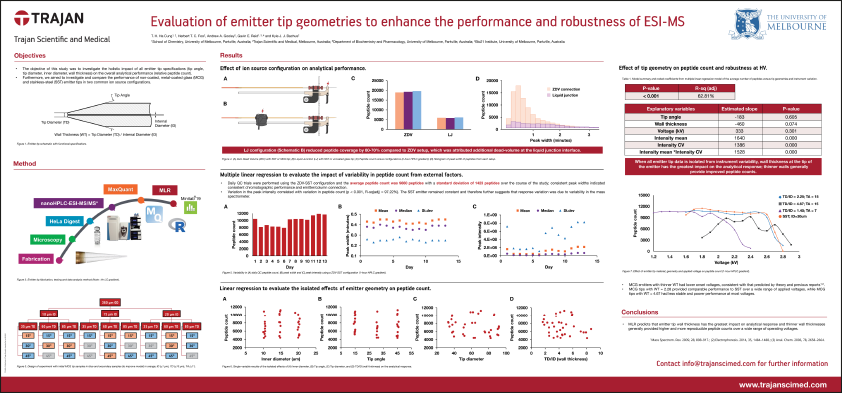
Technical Poster - Evaluation of emitter tip geometries to enhance the performance and robustness of ESI-MS
Collections: All, Analytical, Automation, Literature - All, Literature - Analytical, Literature - Automation, Select by instrument - Analytical, Support items, Technical Posters, Trajan tapered nanospray emitters
Poster - Periodic table of elements and fundamental physical constants
Collections: All, Analytical, Literature - All, Literature - Analytical, Select by instrument - Analytical, Support items, Technical Posters

Technical Poster - Trajan’s LEAP HDX platform is the leading automation solution for HDX-MS applications
Collections: All, Analytical, Automation, CHRONECT™ PAL accessories, CTC Analytics, Literature - All, Literature - Analytical, Literature - Automation, ProDx columns, Select by instrument - Analytical, Support items, Technical Posters
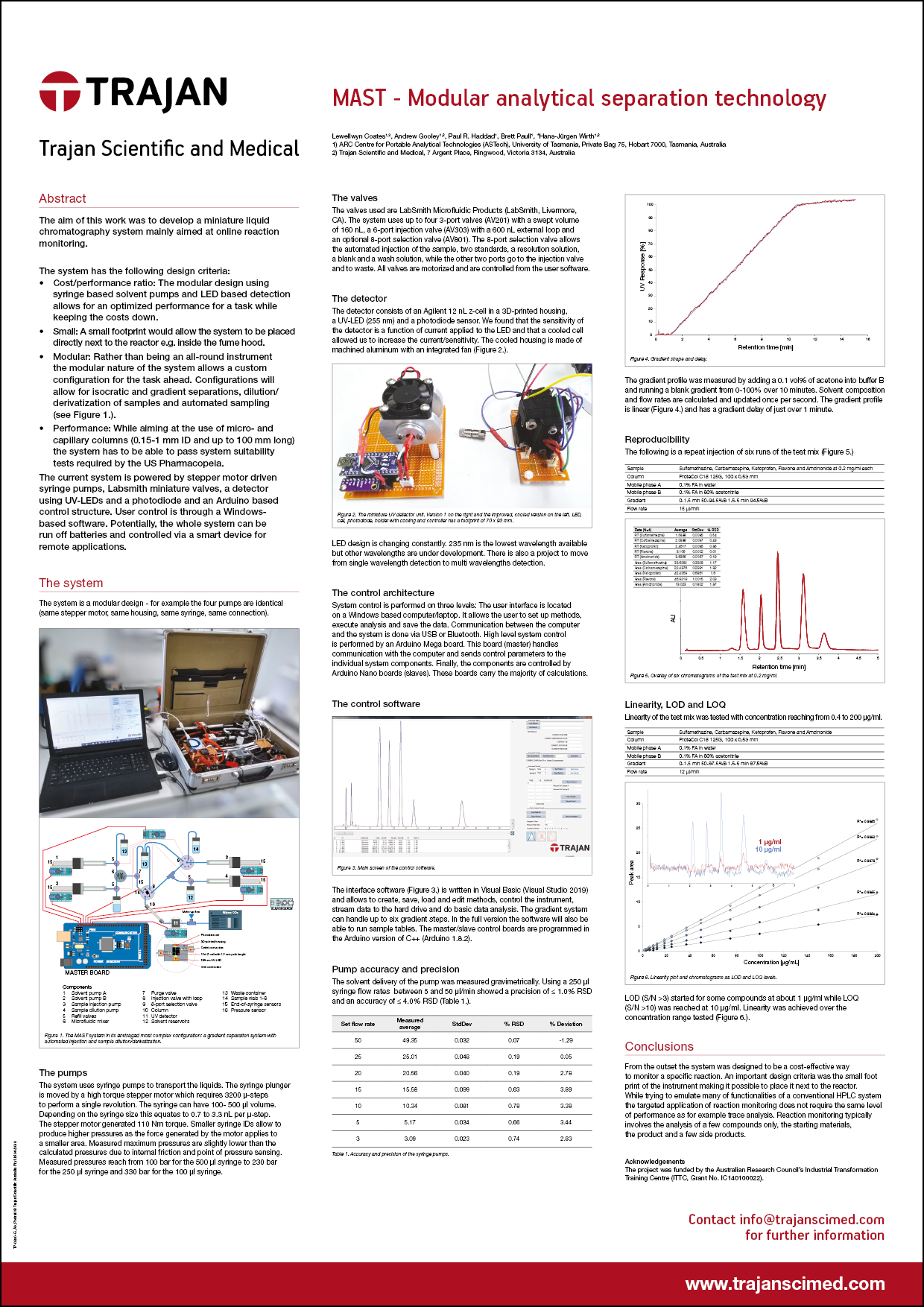
Technical Poster - MAST - Modular analytical separation technology
Collections: Agilent Technologies, All, Analytical, Automation, Literature - All, Literature - Analytical, Literature - Automation, Select by instrument - Analytical, Support items, Syringes, Technical Posters
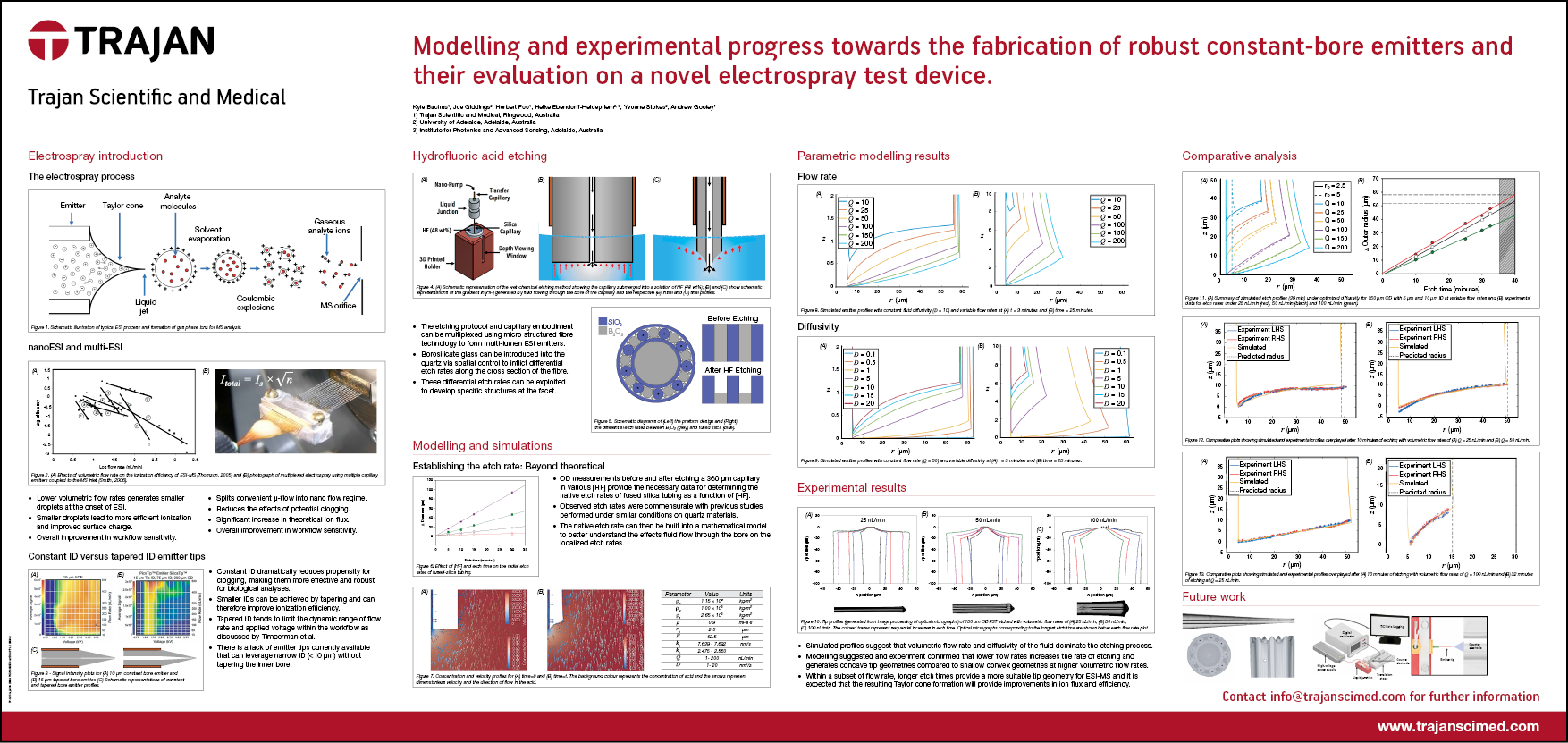
Technical Poster - Modelling and experimental progress towards the fabrication of robust constant-bore emitters and their evaluation on a novel electrospray test device
Collections: All, Analytical, Automation, Literature - All, Literature - Analytical, Literature - Automation, Select by instrument - Analytical, Support items, Technical Posters, Trajan tapered nanospray emitters
Technical Poster - Automated micro liquid-liquid extraction for environmental analysis
Collections: All, Analytical, Automation, CHRONECT™ PAL accessories, CTC Analytics, Literature - All, Literature - Analytical, Literature - Automation, Select by instrument - Analytical, Support items, Technical Posters

Technical Poster - Automated Hydrogen-Deuterium Exchange with optimized consumables
Collections: All, Analytical, Automation, CHRONECT™ PAL accessories, CTC Analytics, Literature - All, Literature - Analytical, Literature - Automation, ProDx columns, Select by instrument - Analytical, Support items, Technical Posters