- All
- Technical Poster - Automated micro liquid-liquid extraction for environmental analysis
+Shop
+Analytical products
+Sampling products
+Pathology products
+Automation systems
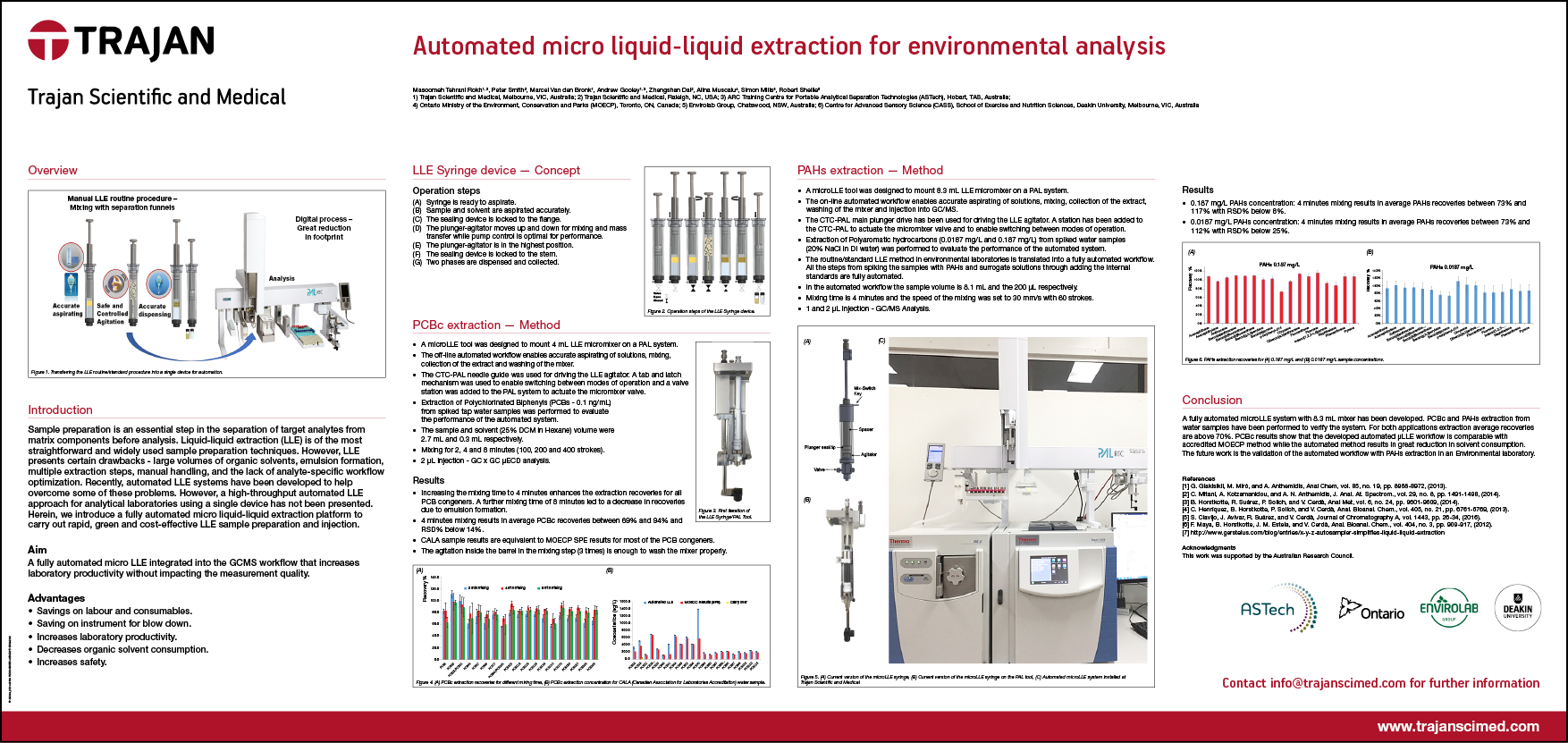
Technical Poster - Automated micro liquid-liquid extraction for environmental analysis
Image is representative (may not be specific item).
Collections: All, Analytical, Automation, CHRONECT™ PAL accessories, CTC Analytics, Literature - All, Literature - Analytical, Literature - Automation, Select by instrument - Analytical, Support items, Technical Posters
Category: Analytical, Automation, Automation consumables, Automation systems, Connectors, CTC, CTC Analytics, HDX, HPLC, LCMS, LEAP, Liquid-liquid extraction, LLE, PAH, PAL, PCB, R&D, Research, Support
Type: Technical Posters
